Harvard-Trained Inflammation Researcher Confirms Plausibility of 49ers EMF Injury Hypothesis
Two well-documented mechanisms. The biochemistry of oxidative stress, collagen degradation, and why the 49ers EMF soft-tissue injury hypothesis demands investigation
“Collagen breakdown does come from inflammatory factors.” — Dr. Christina Gavegnano, Emory University
That sentence was not spoken by an EMF researcher, a biohacker, or a wellness practitioner with a gaussmeter. It came from Dr. Christina Gavegnano—Assistant Professor of Pathology, Pharmacology, and Chemical Biology at Emory University School of Medicine. Dr. Gavegnano’s authority on systemic inflammation is world-class: she holds a PhD in Pharmacology from Emory, where her foundational research on JAK inhibitors provided a breakthrough in blocking the inflammatory pathways that allow HIV to persist in the body. This work directly led to her role as the co-inventor of a repurposed COVID-19 therapeutic (Baricitinib) that earned full FDA approval and conferred a 46% reduction in mortality in critically ill patients.
Outside of the lab, she holds a Master’s in Bioethics from Harvard Medical School and serves as a co-principal investigator on multiple NIH-funded Phase 2 and Phase 3 human clinical trials. When Chase Senior asked her about the substation EMF theory during Super Bowl week, she did not dismiss it. She engaged with it:
“Any number of things that our bodies are exposed to has the potential to be detrimental. You kind of grow up hearing ‘Don’t live near the power lines if you can help it.’ That kind of thing. And you know, I can’t say for sure about the theory, but I would say overarchingly, anything that is extra that you’re exposed to long term is probably not a good thing. I had not heard that. Collagen breakdown does come from inflammatory factors, from, you know, exposure to another series of things, so I would be very curious to look at the data, and the stats on that. You know, see that hard data, because that’s a very interesting concept there.”
Dr. Gavegnano’s response is significant for what it contains and what it lacks. There is no reflexive dismissal. No appeal to regulatory limits. No hand-waving about ionizing versus non-ionizing radiation. Instead, there is an immediate recognition of the mechanistic plausibility: collagen breakdown comes from inflammatory factors, and chronic environmental exposures can supply those factors. What she wants to see is the data connecting the specific exposure to the specific downstream effect.
That data exists, and the bridge between EMF exposure and collagen degradation does not require a single purpose-built study—because it runs through one of the most thoroughly documented processes in all of pathology.
Note: I was assisted in the development of this article by a biochemist whose research focused on 2nd-generation quinolones. He found the EMF hypothesis compelling because the molecular pathways of tendon damage observed in his research lined up with it so well. I want to clarify that I am not a biochemist myself; synthesizing this complex biological work has been a stretch, and if I have mischaracterized any technical details, I welcome feedback to help further this important discussion.
Many critics have argued that the 49ers EMF induced soft-tissue injury hypothesis lacks a single, direct study proving that electromagnetic fields degrade collagen fibers. I believe it is important to pursue this line of research, and as such I have partnered with ResearchHub to help raise $100k to fund up to eight studies on the connection between EMF and collagen degrdation. However, this demand for a “smoking gun” study overlooks how biology actually functions. We don’t need a specialized study for every individual environmental stressor if we can establish a mechanistic bridge through an undisputed, non-controversial biological process.
We can do that and the process is known as oxidative stress—and it is exactly the kind of "inflammatory factor" Dr. Gavegnano was referencing. Oxidative stress and inflammation are not separate phenomena; they are two faces of the same biological response. When cells are damaged by reactive molecules, the body treats it the same way it treats an infection or a wound—it mounts an inflammatory response. The difference is that the trigger here is not a pathogen or a torn muscle fiber, it is a chronic environmental exposure.
The Undisputed Foundation: Oxidative Stress
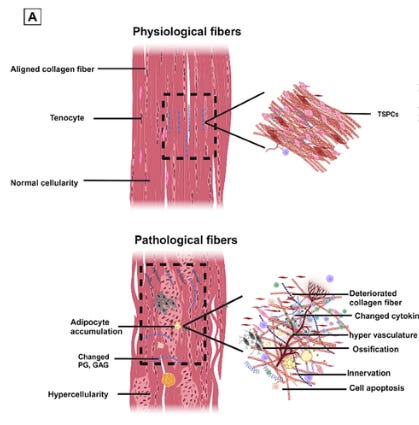
Oxidative stress is not a fringe theory; it is a fundamental pillar of pathology. It occurs when the production of Reactive Oxygen Species (ROS)—unstable molecules that damage cell structures—overwhelms the body’s antioxidant defenses.
This mechanism is non-controversial and is the recognized driver behind a vast array of diseases:
Cardiovascular Disease: ROS-driven oxidation of LDL cholesterol leads to arterial plaque.
Neurodegenerative Disorders: Oxidative damage is a primary culprit in Alzheimer’s and Parkinson’s.
Diabetes: ROS contribute to insulin resistance and damage to pancreatic cells.
Cancer: ROS cause direct DNA strand breaks and mutations, acting as a key driver of oncogenesis.
Oxidative Stress and Collagen Degradation
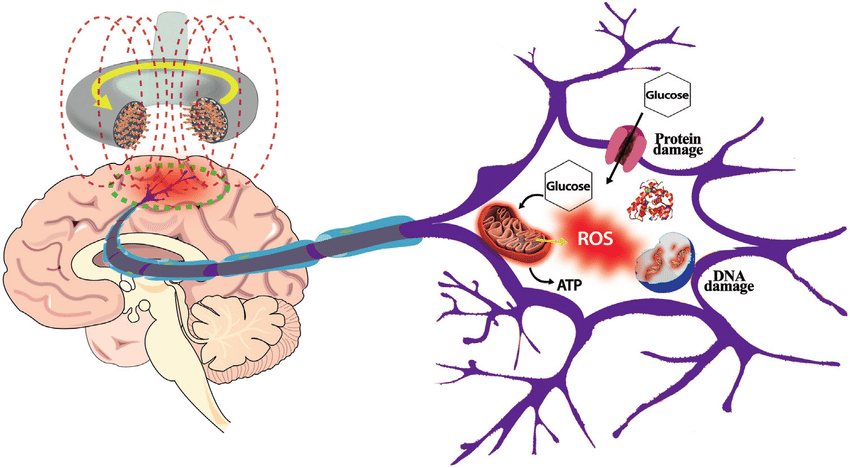
Once a biological system experiences an influx of ROS, the body's internal "collagen shredding" machinery—the Matrix Metalloproteinases, or MMPs—is activated. MMPs are themselves products of inflammatory signaling driven by the same ROS. This is a well-documented biochemical pathway.
MMP Activation: Oxidative stress activates transcription factors like AP-1, which acts as a master “on-switch” for MMP-1.
Fragmentation: MMP-1’s specific role is to seek out and cleave the collagen triple helix, leading to structural fragmentation.
The Positive Feedback Loop: A landmark study in The American Journal of Pathology found that fragmented collagen itself promotes further oxidative stress in fibroblasts, creating a self-perpetuating cycle of breakdown.
Synthesis Inhibition: Simultaneously, ROS inhibit the synthesis of new collagen by downregulating Type I procollagen, leading to a net loss of tissue integrity.
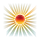
Why Tendons Are Particularly Vulnerable
While this mechanism is often discussed regarding skin aging (wrinkles and photoaging), it is even more critical for tendons. Because tendons have a lower blood supply and slower metabolic turnover than the dermis, they are less capable of repairing the damage caused by this oxidative stress.
Furthermore, ROS directly modify the amino acids histidine and lysine within the collagen structure. These oxidative modifications impact the tendon’s mechanics, making the tissue brittle and prone to failure rather than healthy stretching.
Distinguishing Damage from Environmental Factors vs Wear and Tear?
Research into tendon pathology differentiates between acute mechanical overload (sports injuries) and degenerative tendinopathy driven by oxidative stress. While sports injuries often involve physical “fraying” or macroscopic tears, ROS-mediated damage manifests as an underlying biochemical and structural reorganization of the tissue.
The following factors explain why ROS-mediated damage creates a more “irregular” appearance:
1. Dysfunctional Cross-Linking and Brittleness: In a healthy tendon, collagen fibers are organized in highly parallel, crystalline arrays held together by precise enzymatic cross-links.
ROS Modification: Oxidative stress targets the lysine and histidine residues of the collagen molecule.
Irregularity: This creates “Advanced Glycation End-products” (AGEs) and dysfunctional cross-links that disrupt the parallel alignment. Instead of the long, smooth waves (crimping) seen in healthy tendons, the fibers become “kinked” and brittle.
2. The “Non-Collagenous” Shift: When ROS levels remain high, the cells stop producing high-quality Type I collagen and start producing “filler” materials.
Matrix Overproduction: To compensate for the “shredding” caused by MMPs, the body deposits disorganized Type III collagen.
Visual Difference: Under a microscope, sports-damaged tendons may show clear break lines, but ROS-damaged tendons show “chondroid metaplasia”—where the tendon starts looking less like a rope and more like irregular, bubbly cartilage.
3. Diffuse vs. Localized Fragmentation:
Sports Damage: Usually localized to the site of the tear or highest mechanical strain.
ROS Damage: Because oxidative stress can be systemic or triggered by external sources the degradation is often diffuse. The entire matrix begins to look “moth-eaten” or “irregular” because the MMP enzymes are attacking the fibers from the inside out throughout the tissue.
4. Tenocyte Morphological Changes: In sports injuries, the cells are often trying to repair the gap. In ROS-mediated damage, the cells themselves become “stressed” and irregular in shape.
Apoptosis: High ROS levels lead to tenocyte death.
Acellular Gaps: This leaves “holes” in the tissue where no repair is happening, contributing to the irregular and thinning look of the collagen matrix.
The Final Link: EMFs as a Trigger for Oxidative Stress
If we establish that EMFs trigger oxidative stress, we have the second half of our bridge. Multiple independent research teams have systematically reviewed this evidence:
Dr. Henry Lai (Professor Emeritus, University of Washington) has compiled the most comprehensive collection of peer-reviewed research on EMF bioeffects. His findings:
Radio Frequency Radiation (RFR): 89% of 407 studies published since 1997 reported significant oxidative effects.
Extremely Low Frequency (ELF) EMF: 90% of 332 studies since 1990 reported significant oxidative or free radical effects.
Low-Intensity Impact: 97% of 102 studies with a Specific Absorption Rate (SAR) ten times lower than FCC safety limits still showed significant oxidative stress.
Yakymenko et al. (2016) reviewed 100 peer-reviewed studies examining oxidative effects of low-intensity RFR in living cells. Their conclusion: 93 of 100 studies confirmed that RFR induces oxidative effects, including activation of key ROS-generating pathways, DNA damage, and changes in antioxidant enzyme activity. They concluded that “low-intensity RFR is an expressive oxidative agent for living cells with a high pathogenic potential.”
Schuermann & Mevissen (2021) conducted a comprehensive review of animal and cell studies from 2010-2020, finding that “most animal and many cell studies showed increased oxidative stress caused by RF-EMF and ELF-EMF”—including at exposure levels below current regulatory limits.
Li & Héroux (2014) exposed five cancer cell lines to ELF magnetic fields (0.025–5 µT) and found a "mostly flat dose-response"—demonstrating that biological effects occur across a wide range of field strengths rather than requiring a threshold intensity. Crucially, they discovered an adaptation-and-perturbation mechanism: constant exposures allowed cells to return to baseline over three weeks, after which even small field increases or decreases re-triggered the effects. This suggests the body responds not to absolute field strength, but to changes in the electromagnetic environment—explaining why regulatory frameworks focused solely on intensity thresholds may miss biologically relevant exposures.
5: Confounding Factors
When examining professional athletes, we must acknowledge they are subjected to unusual levels of mechanical force that put extreme strain on tendons and ligaments. I am not suggesting that EMFs are the sole factor in these injuries, but rather a significant environmental stressor that tips the biological balance toward failure in the case of the 49ers due to their proximity to the Mission substation. Athletes are often subject to other confounding environmental factors that utilize the exact same ROS-MMP pathway:
Fluoroquinolone Antibiotics (e.g., Ciprofloxacin, Levofloxacin): This specific class of antibiotics—commonly known by brand names like Cipro or Levaquin—is notorious in sports medicine for its black box warning regarding tendon rupture. These drugs trigger a burst of ROS and upregulate MMPs, directly destabilizing the collagen matrix.
Likelihood of Use in Athletes (MRSA & Skin Infections): Professional athletes, particularly in contact sports like football, are at a higher risk for MRSA (Methicillin-resistant Staphylococcus aureus) and other stubborn skin or soft-tissue infections due to locker room environments and turf burns. Fluoroquinolones are powerful, broad-spectrum tools often used when other antibiotics fail, unintentionally placing the athlete’s tendons at risk.
Corticosteroids: While frequently used to manage acute inflammation so a player can return to the field, these can inhibit collagen synthesis and alter the mechanical properties of the matrix.
The “CMC” Case Study (Bilateral Tendinitis): The presence of bilateral Achilles tendinitis, as seen in the case of Christian McCaffrey, the star running back for the 49ers, often points away from isolated wear and tear injuries—which are typically unilateral—and toward systemic triggers.
Conclusion: The Chain is Complete
We do not need to wait for a study titled “EMF Degrades Collagen” to recognize a significant biological risk. Complex pathology rarely offers a single smoking gun. What it offers is convergence—multiple established mechanisms feeding into the same downstream outcome through a shared pathway.
Environmental Trigger: Consistent evidence across multiple systematic reviews demonstrates that EMF exposure increases the body’s oxidative load by driving production of Reactive Oxygen Species.
Biochemical Pathway: It is an undisputed medical fact that ROS-driven oxidative stress activates Matrix Metalloproteinases (MMPs)—the enzymes responsible for cleaving and degrading the collagen triple helix.
Resulting Vulnerability: Chronic exposure keeps the collagen-shredding machinery in a state of constant low-level activation—not enough to cause outright failure on its own, but enough to lower the biological threshold at which failure occurs.
A standard athletic load that any healthy tendon should absorb becomes a catastrophic event when the tissue has been quietly degraded from the inside for months or years.
The EMF is an important but currently overlooked factor that frays the tendon before the loads on the playing field are ever applied. And when you account for the slow repair cycle of tendon tissue—far slower than skin, far slower than muscle—that continuous oxidative pressure becomes one of the most consequential variables in an athlete’s structural durability.
The evidence meets the threshold for serious concern. Players may have multi-millionaire contracts, but their bodies become a commodity when they sign that contract, and their health is important in and of itself. We know from the past that the NFL is prone to sweeping injury risk under the rug, as with the case of concussions and Chronic Traumatic Encephalopathy (CTE). But CTE is a progressive degenerative brain disease that affects players lives after they have left the playing field. This collagen degradation affects them while they are still on the field, and hurts the teams ability to stay healthy and win. Keeping players healthy is the teams highest priority, so in this case what is the downside of taking precautions?
The mechanistic bridge is built from established science on both sides—consistent oxidative stress findings from EMF across dozens of systematic reviews, and an uncontested downstream pathway from ROS through MMP activation to collagen degradation.
This does not prove EMFs cause tendon injuries. But it establishes sufficient mechanistic plausibility that dismissing the hypothesis requires explaining away a substantial body of evidence.
Dr. Gavegnano put it plainly:
“That’s one of the things about these new theories is that we don’t know quite what is the mechanism that’s driving it. And you start to see if something is real? Maybe it’s huge and 20 years later we look back and say ‘Are you kidding me? I can’t believe we did that.’ But we don’t know quite yet, so it’s something to keep an eye on, for sure.”
Twenty years from now, the question won’t be whether the science was available. It will be whether anyone acted on it.
Supporting Literature
Fisher, G. J., et al. (2009). Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. The American Journal of Pathology.
Xia, W., et al. (2013). Expression of catalytically active matrix metalloproteinase-1 in dermal fibroblasts induces collagen fragmentation and functional alterations. Aging Cell.
Qin, Z., et al. (2014). Oxidant Exposure Induces Cysteine-Rich Protein 61 (CCN1) via c-Jun/AP-1 to Reduce Collagen Expression in Human Dermal Fibroblast. PLoS ONE.
Shroff, A., et al. (2014). Oxidative Stress and Skin Fibrosis. Current Pathobiology Reports.
Wang, T., et al. (2024). Role of oxidative stress in the concurrent development of osteoporosis and tendinopathy. Journal of Cellular and Molecular Medicine.
Lui, P. P. Y., et al. (2022). Roles of Oxidative Stress in Acute Tendon Injury and Degenerative Tendinopathy. International Journal of Molecular Sciences.
Chen, X., et al. (2021). Research progress on skin photoaging and oxidative stress. Advances in Dermatology and Allergology.
Moriggi, M., et al. (2024). Reactive oxygen species in tendon injury and repair. Redox Biology.
Reiser, K. M., et al. (2012). UV Damage of Collagen: Insights from Model Collagen Peptides. Biopolymers.
Lai, H. (2025/1990). Exposure to Static and Extremely-Low Frequency Electromagnetic Fields and Cellular Free Radicals. Electromagnetic Biology and Medicine / University of Washington Abstracts.
National Toxicology Program (NTP). (2018). Technical Report 595: Toxicology and Carcinogenesis Studies in Sprague Dawley Rats Exposed to Whole-body Radio Frequency Radiation. U.S. Department of Health and Human Services.
Yakymenko, I., et al. (2016). Oxidative mechanisms of biological activity of low-intensity radiofrequency radiation. Electromagnetic Biology and Medicine.
Schuermann, D., & Mevissen, M. (2021). Manmade Electromagnetic Fields and Oxidative Stress—Biological Effects and Consequences for Health. International Journal of Molecular Sciences.
Burlaka, A., et al. (2013). Overproduction of free radicals in embryonic cells under low-intensity radiofrequency radiation. Experimental Oncology.
Li, Y., & Héroux, P. (2014). Extra-low-frequency magnetic fields alter cancer cells through metabolic restriction. Electromagnetic Biology and Medicine.
Tanne, J. H. (2008). FDA adds “black box” warning label to fluoroquinolone antibiotics. The BMJ.
Bacon, C. E. W., et al. (2020). Skin and Soft Tissue Infections in Athletes. Current Sports Medicine Reports.
Gocan, S. B., et al. (2023). Overview of Side-Effects of Antibacterial Fluoroquinolones: Underlying Mechanisms of Tendinopathy. Pharmaceutics.