How Low-Level Electromagnetic Fields Disrupt Mitochondrial Function and Trigger Cellular Dehydration: The Hidden Pathways to Collagen Fragility
In Part 1, we established the 49ers’ decade-long injury outlier—unmatched soft-tissue failures no other team replicates—and pinpointed the unique variable: chronic ELF magnetic fields from the Metcalf substation next door, measured at biologically active levels (8 mG at minimum, potential up to 40+ mG at peak) across their practice facilities.
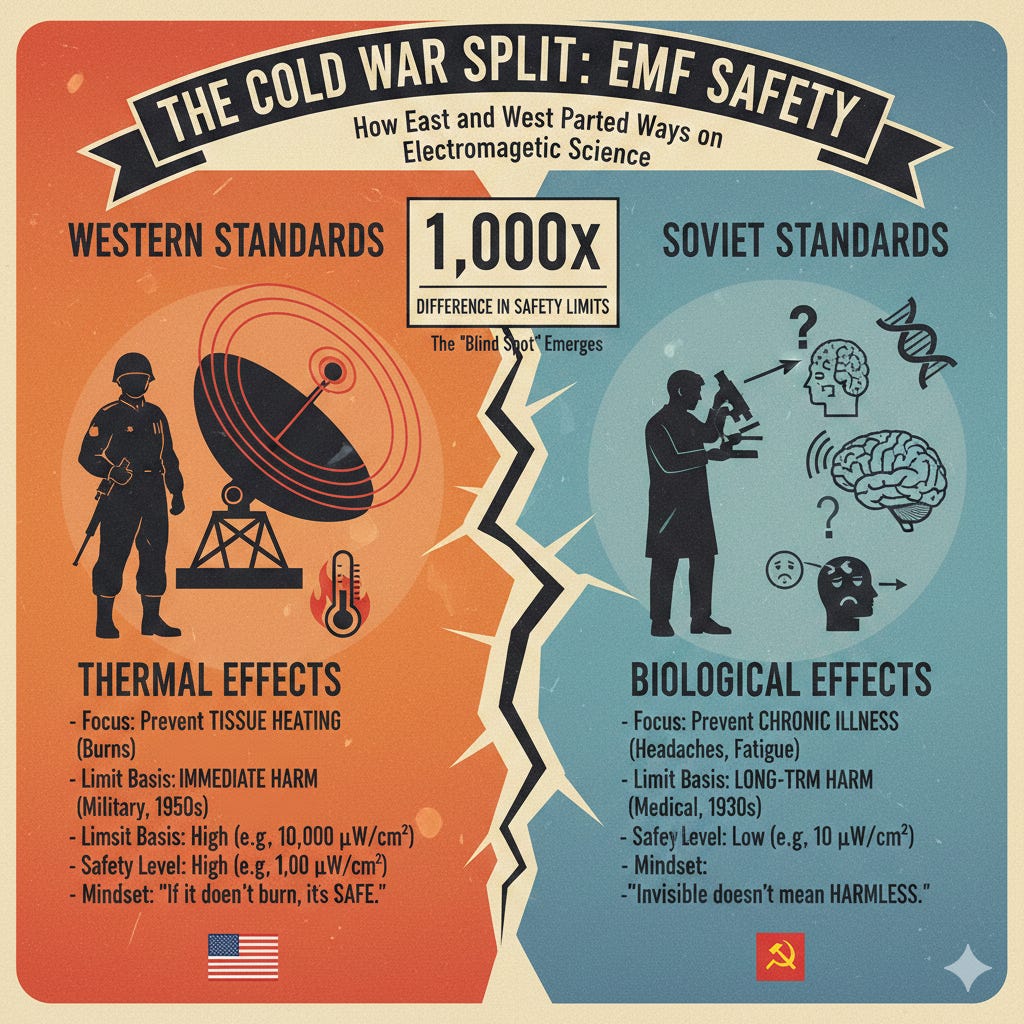
If these non-thermal effects are backed by thousands of studies, why do official guidelines insist there’s no risk? The answer lies in an outdated “heat or nothing” safety paradigm—one that traces straight back to Cold War geopolitics and still blinds regulators, engineers, and team officials today.
The Cold War Split: How East and West Parted Ways on EMF Safety
To understand why the “heat or nothing” rule persists, you have to go back to the Cold War, when the U.S. and Soviet Union turned electromagnetic research into a battleground.
Western standards were shaped by American military engineers in the 1950s–60s who were worried about radar operators literally getting cooked. They set the first exposure limits at levels that prevented measurable tissue heating (about 10 mW/cm², later translated into SAR limits for phones and milligauss limits for power-frequency fields). Anything below that threshold produced no immediate burns or shocks, so it was declared “safe.” When the FCC adopted those military numbers in 1996 and ICNIRP followed in 1998, the thermal-only paradigm became the standard, ignoring subtler biology entirely.
The Soviets, however, took a different path. Their scientists, studying non-thermal effects since the 1930s, documented headaches, fatigue, and immune changes from low-level microwaves in workers. By the 1970s, they set exposure limits 1,000 times stricter than the West’s, based on chronic low-dose harm.
This divergence exploded in 1976 when U.S. intelligence discovered the “Moscow Signal”—Soviets beaming low-power microwaves (5 µW/cm², non-thermal) at the U.S. Embassy for 23 years. Intended as jamming or harassment, it sparked “microwave sickness” reports: fatigue, vision issues, and elevated cancers among staff. Ambassador Walter Stoessel developed leukemia and died in 1986; three ambassadors total succumbed to cancers linked to the exposure. Secretary Henry Kissinger, briefed in 1975, quashed hazard pay for all embassy workers to avoid embarrassing détente, despite internal memos calling it a health hazard. The incident fueled U.S. secrecy (Project Pandora) but reinforced the thermal-only dogma—dismissing Soviet non-thermal data as propaganda, even as declassified studies later showed leukemia clusters 2–3x expected rates.
That geopolitical blind spot lingers: Western guidelines still prioritize heating over the biology the East warned about decades ago, but despite this blind spot, and institutional inertia, research on non-thermal effects have progressed, even in the West.
What follows is the step-by-step science—none of it speculative, all of it published, peer-reviewed research—that explains exactly how a humming substation adjacent to the practice facilities can turn elite athletes in the high tech capital of the country into the league’s most fragile.
Why Collagen Fails Under Stress from AC Magnetic Fields
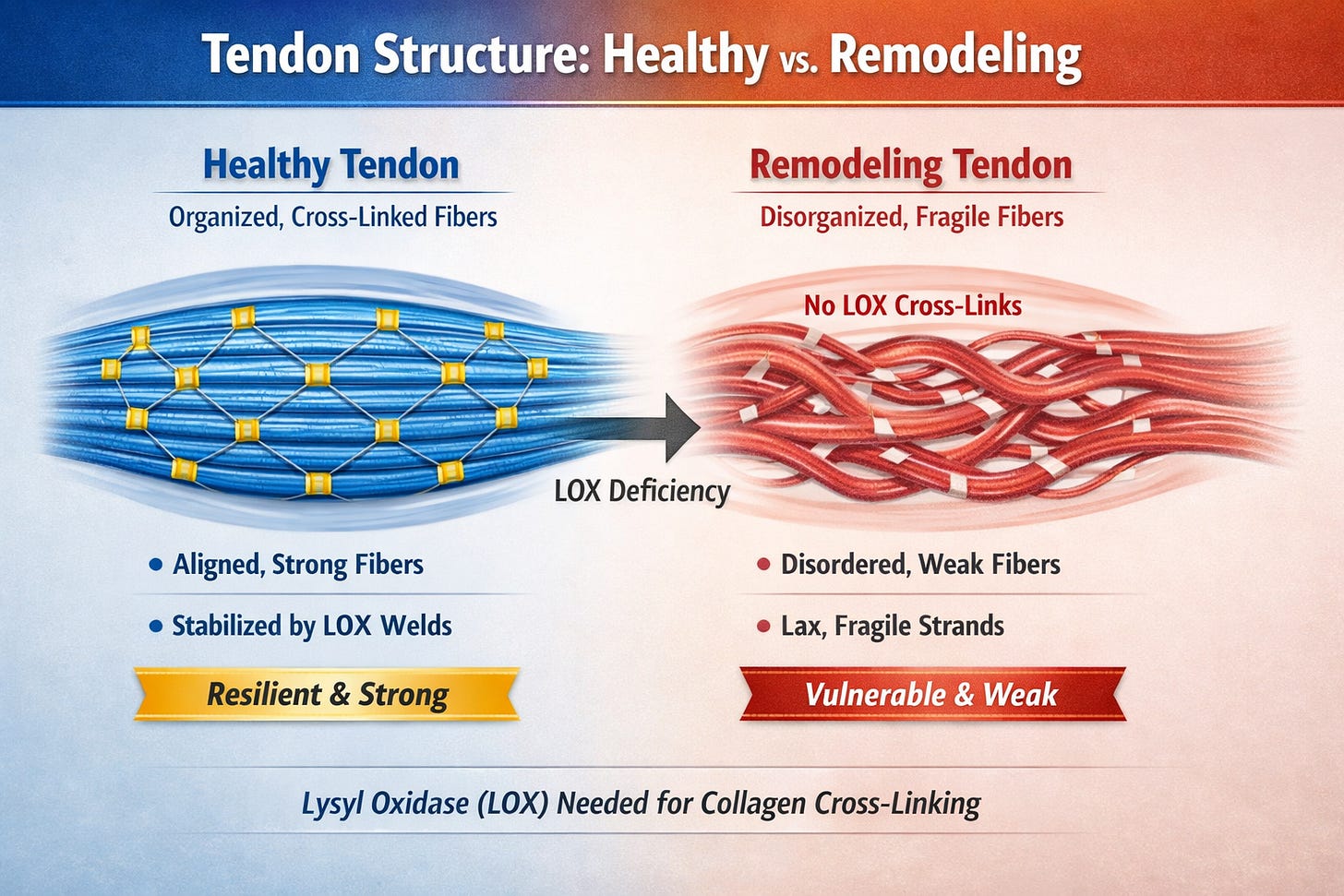
Collagen type I, the rope-like protein that gives tendons their tensile strength (up to 10,000 psi), is not a passive, inert cable; it’s a dynamic, electrically sensitive structure. Pioneering bioelectromagnetics researchers Robert Becker and Andrew Marino demonstrated as early as the 1970s that collagen exhibits piezoelectric properties–meaing it converts mechanical stress into electrical signals that govern tissue repair. Conversely, recent biophysical models suggest that when external, man-made fields override these internal signals, they can disrupt the 'crystalline lattice' of the collagen fibers. While Becker focused on the healing potential of these fields, subsequent research has explored how chronic, low-level exposure can interfere with collagen synthesis, leading to the structural vulnerabilities seen in soft-tissue failures.910
Fast-forward to today: Modern research confirms that the magnetic fields pouring out of the Metcalf substation—precisely in this 8–50 mG window—trigger a multi-pathway assault on collagen via non-thermal mechanisms. The first domino falls with mitochondrial dysfunction.
Pathway 1: Mitochondrial Dysfunction and Cellular Dehydration
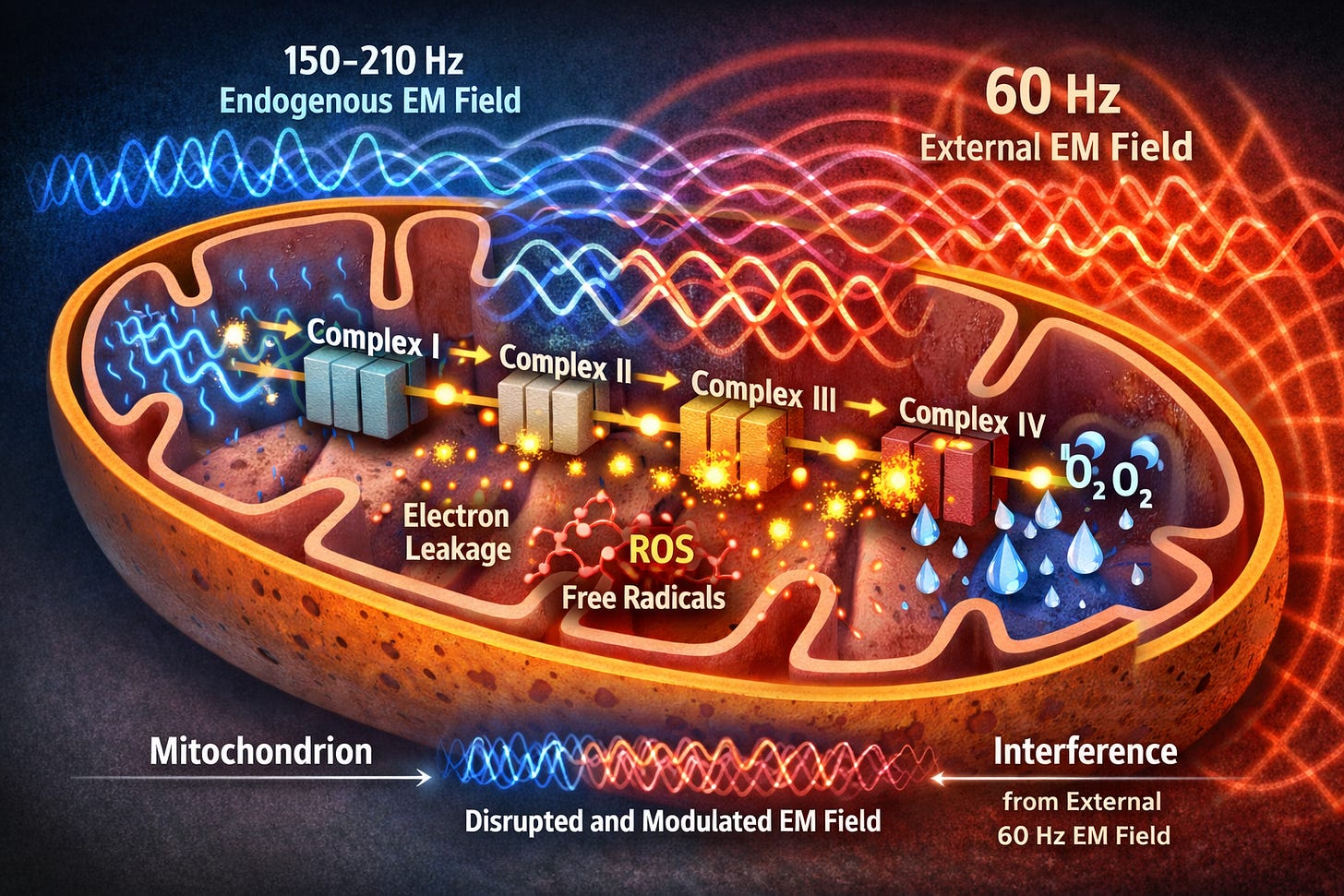
To understand the larger problem, let’s first zoom into how cells generate energy: the mitochondrion. Mitochondria are microscopic organelles found by the thousands in nearly every cell, and the site where the Krebs cycle and electron transport chain turn nutrients and oxygen into usable energy through a process called oxidative phosphorylation.3,11 At its core is the electron transport chain (ETC), a series of four protein complexes embedded in the inner mitochondrial membrane. Think of the ETC as an assembly line powered by high-energy electrons derived from nutrient substrates (carbohydrates, fats, and amino acids). These electrons cascade down the chain—Complex I to II to III to IV—while the complexes pump protons (hydrogen ions) across the membrane, building the proton motive force (PMF): These forces act like a battery with the negative (electrons) on one side, and the positive (protons) on the other, creating a mitochondrial membrane potential (delta psi) of –140 to –180 millivolts.
While that voltage sounds small, it exists across a membrane only 5 to 7 nanometers thick; this results in an internal electric field strength of approximately 30 million volts per meter—an electrical intensity equivalent to a bolt of lightning. This delta psi is the cell's real energy currency; it spins a tiny biological turbine (ATP synthase) to crank out adenosine triphosphate (ATP), the universal fuel for everything from muscle contraction to collagen cross-linking.
A tiny amount of electron leakage in this process is completely normal; it produces small bursts of reactive oxygen species (ROS) that act as cellular signals, communicating the health of the cell. Cytochrome c oxidase (COX), Complex IV in the ETC is the grand finale, where those electrons hand off to oxygen molecules, forming metabolic water—up to 32 water molecules for every glucose molecule the cell consumes. This structured “exclusion zone” (EZ) water hydrates proteins like collagen, keeping them supple and elastic.
But here’s the twist: As electrons tunnel between complexes, the ETC generates its own weak endogenous electromagnetic field—oscillating at 150–210 Hz.3 When an external 60 Hz magnetic field from the substation occupies the same space as the mitochondrion’s own much weaker, higher-frequency field, basic physics takes over. Maxwell’s equations are the four simple rules of physics that govern all electricity and magnetism—rules so fundamental that every light bulb, radio wave, and power line–man made or naturally occurring–obeys them without exception. One of those rules says: when two electromagnetic fields meet in the same space, they just overlap and add together, combining into one new, less coherent wave pattern.
In recent work (Héroux, 2024/25), it is proposed that the superposition of these 60 Hz fields interferes with quantum-level electron transfers via a process known as the radical-pair mechanism. Essentially, the magnetic field alters the spin states of electrons as they move through the mitochondria; by keeping these electrons in a spin-correlated state longer than normal, the field increases the probability that they will react with nearby oxygen to form ROS rather than completing their intended path. By disrupting the precise timing of these transfers, the fields act as a form of signal noise that knocks electrons off course. To visualize this, picture a fast, delicate ripple (the mitochondrion’s natural high-frequency 150-210 Hz rhythm) riding on top of a slow, heavy swell (the substation’s 60 Hz field). The stronger 60 Hz wave stretches and warps that delicate ripple, creating “signal noise” that throws off the precise timing of the electron transport chain.
Electrons that normally follow a clean, shielded path now have to push through this warped field. Many get knocked off course or spill out sideways instead of completing the precise hand-off at Complex IV. That’s all it takes. The superposition alone is enough to throw the timing off, and the result is that far more electrons leak prematurely than nature ever intended. Those normal, low-level ROS signals explode into an avalanche of oxidants—especially peroxynitrite—that damage proteins, destroy membrane lipids, and slash ATP production4,6 At the same time, far less metabolic water is produced, so collagen dries out from the inside and loses its elasticity.
While the fallout of the interference from the 60hz field leads to the cellular damage described above, there is a stealthier effect from the cellular dehydration. When mitochondria are sufficiently damaged that they produce more ROS than ATP, the cell is forced to fall back on glycolysis—burning sugar directly in the cytoplasm. Glycolysis is far less efficient (only 2 ATP per glucose instead of ~32 from full oxidative phosphorylation) and yields zero metabolic water. Worse, it ends with the production of lactic acid, which lowers local pH and creates a mild acidosis. Collagen is exquisitely sensitive to pH: even a small drop in alkalinity weakens the hydrogen bonds that hold the triple helix together and reduces the water-binding capacity of its hydrophilic side chains. The result is a double hit—less water made, more acid produced—so the collagen fibrils lose their gel-like hydration, stiffen, and become far more brittle. Tendons that should feel like steel cables quietly turn into old rope—one explosive movement away from catastrophic failure, exactly the pathology seen in 49ers Achilles ruptures.4,6,11
But the mitochondrial collapse is only half the battle. While these organelles are failing from within, the cell’s outer defenses are also being breached.
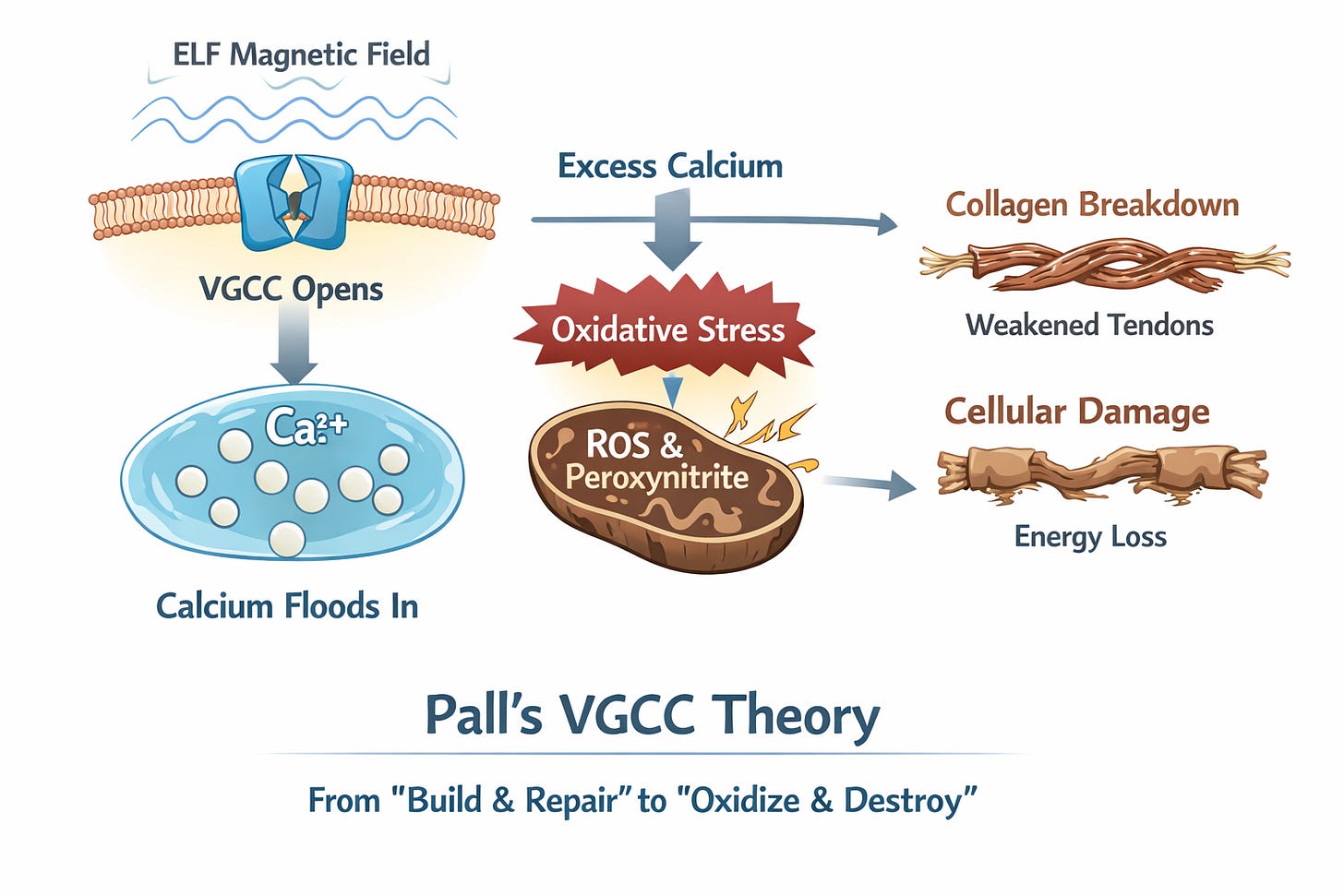
Pathway 2: The Calcium Flood
Biophysicist Martin Pall has spent two decades mapping how ELF magnetic fields activate VGCCs on cell membranes. Unlike thermal effects (which require 1,000+ mG), non-thermal ELF in the everyday 1–100 mG range rotates VGCC proteins just enough to open them, flooding cells with calcium ions.1,2
The sensitivity of these channels lies in their voltage sensor, which is over seven million times more sensitive to electrical forces than the rest of the cell membrane. Pall’s research shows that because the voltage sensor is located in the fatty, non-conductive layer of the membrane, it amplifies the force of an external field, allowing even the weak 60 Hz hum of a substation to exert enough force to keep the gate stuck open.
That excess calcium sets off a chain reaction. In fibroblasts (the cells that make collagen), the overload boosts nitric oxide production. Nitric oxide then collides with the superoxide already leaking from stressed mitochondria and forms peroxynitrite—a particularly nasty oxidant that acts like cellular acid: it breaks proteins apart and degrades the extracellular matrix that holds everything together.
Pall’s VGCC theory ties this directly to the older experiments. McLeod and Lee’s landmark study (published in Science, 1987) provides the missing link: they demonstrated that low-level ELF fields—specifically at the 60 Hz power frequency—can cut fibroblast protein and collagen synthesis by up to 30%. While earlier researchers saw these effects without understanding why, Pall’s model explains the 'how': the fields open VGCCs, calcium floods in, and the whole cellular factory is thrown into oxidative disrepair, slowing the assembly of new collagen strands.17
The same flood of calcium pours into mitochondria, forcing open an emergency “blow-out valve” called the permeability transition pore. This causes the electrical gradient to collapse, effectively shorting out, and a torrent of ROS unleashed, amplifying the oxidative stress described in Pathway 1.
To break it down simply: normal calcium signals “build and repair.” Too much screams “oxidize and destroy”—scarring the collagen scaffolding while crippling the energy factories that keep tissues alive. Over months, tendons that should be steel cables become more like frayed string.
These two pathways alone—mitochondrial sabotage and calcium flooding—paint a picture of relentless cellular damage, but the assault doesn’t end here. The fields also turn the body’s own immune guardians against it, triggering chronic low-grade inflammation and betrayals that remodel connective tissue into something far weaker.
Coming tomorrow in Part 3: “How Invisible Fields Trigger Immune Dysfunction and Sabotage Nighttime Recovery: Mast Cell Activation, Immune Suppression, and Circadian Disruption” – we’ll uncover how these fields hijack immunity, suppress recovery, and disrupt the critical overnight processes that rebuild collagen.
Appendix A — Key Studies & Reviews
1. VGCC / Calcium
Primary researcher(s): Martin Pall
Significance: Establishes the mechanism by which ELF fields activate Voltage-Gated Calcium Channels.
2. VGCC review
Primary researcher(s): Martin Pall
Significance: Comprehensive review of EMF-induced calcium signaling and its downstream health effects.
3. Disruption of mitochondrial electron transport chain function potentiates the pro-apoptotic effects of MAPK inhibition
Primary researcher(s): Andrew P Trotta
Significance: Details how ETC dysfunction and electron leakage trigger cellular stress and apoptosis (programmed cell death).
4. ELF magnetic fields & mitochondrial ROS (The ATP Synthase Mechanism)
Primary researcher(s): Paul Héroux (with Ying Li)
Significance: Provides the laboratory evidence that ELF magnetic fields act on the protons within the ATP Synthase (Complex V) enzyme. By interfering with the proton flow that maintains the delta psi membrane potential, the fields create backpressure in the electron transport chain that forces the leakage of ROS.
Source: Héroux, P., & Li, Y. (2014). “Extra-low-frequency magnetic fields alter cancer cells through metabolic restriction.” Electromagnetic Biology and Medicine, 33(4), 264-275.
6. Non-thermal ELF bioeffects via radical-pair mechanism in ETC
Primary researcher(s): Paul Héroux
Significance: The 2024/25 paper explaining the quantum “superposition” and timing disruption of electron transfer.
9. Piezoelectricity as a Fundamental Biological Control Mechanism
Primary researcher(s): Becker, R. O., and Marino, A. A.
Significance: This is the definitive paper establishing that the electrical properties of the extracellular matrix (collagen) are what control growth and repair processes.
Source: Marino, A. A., & Becker, R. O. (1970). “Piezoelectric Effect and Growth Control in Bone.” Nature, 228(5270), 473–474.
10. Piezoelectric properties of collagen (review)
Primary researcher(s): Magda Havas et al.
Significance: Review of the biological effects of EMFs on the crystalline structure of the extracellular matrix.
11. The Role of Cytochrome c Oxidase in Metabolic Water Production
Primary Researcher(s): Mårten Wikström (Standard Science)
Significance: This research details the precise mechanism of Cytochrome c Oxidase (Complex IV) in the electron transport chain. It established that Complex IV not only generates ATP but is the terminal site where oxygen is reduced to form metabolic water, which is essential for cellular hydration.
Source: Wikström, M., & Sharma, V. (2018). Proton Pumping by Cytochrome c Oxidase. Chemical Reviews, 118(14), 6841-6861.
17. Frequency dependence of electric field modulation of fibroblast protein synthesis
Primary researcher(s): K J McLeod, R C Lee, H P Ehrlich
Significance: A seminal study in the journal Science proving that 60 Hz fields (the frequency of the Metcalf substation) specifically inhibit the synthesis of proteins and collagen in fibroblasts.

Brillaint breakdown of the EMF-mitochondrial connection here. The metabolic water angle is something I hadn't considerd before, but it makes total sense when thinking about collagen hydration. I remember reading about glycolysis fallback in cancer cells years ago and this shares that same energy desperation pattern. The pH drop from lactic acid buildup adding to collagen brittleness is kinda underappreciated in most discussions about soft tissue failure.
EXCELLENT article, Peter! I'll save this, and include some excerpts in the health effects section of my white paper. Good work mate.