How Invisible Fields Trigger Immune Dysfunction and Sabotage Nighttime Recovery: Mast Cell Activation, Immune Suppression, and Circadian Disruption
(Part 3 of "The 49ers' Decade of Fragility: How Invisible EMFs Could Be Undoing an Elite Roster" series. If you missed Part 1 or Part 2, catch up with the links below. Subscribe for Part 4 tomorrow.)
In Parts 1 and 2, we established the 49ers’ unique exposure to ELF magnetic fields from the substation next to their practice fields and explained how these fields disrupt the mitochondrial electron transport chain and flood cells with calcium, leading to dehydrated, brittle collagen. But the damage doesn’t stop at cellular energy production and oxidation—there’s an immune mechanism happening too, turning the body’s defenders against its own tissues while robbing cells of the nightly repair they need to stay resilient.
Pathway 3: Mast Cells Activation
Tendons and ligaments are laced with mast cells—immune sentinels that normally stay quiet, packed with chemical weapons meant for real emergencies. Olle Johansson’s group at Karolinska Institute showed that low-level magnetic fields fool these cells into thinking there’s danger. Once that fuse is lit, the consequences are dictated by the body's own chemical arsenal, as detailed by researchers like Millar and Pejler7, they pop open and dump their payload:
Histamine – triggers local swelling and fluid leakage, loosening the tissue matrix.18
Cytokines IL-4 & IL-13 – shift the repair process into a low-grade, chronic inflammatory mode that favours weak, scar-like collagen instead of strong, parallel fibres.19
Proteases (tryptase & chymase) – act like molecular acid, directly chewing up existing collagen and elastin strands.20
VEGF & TGF-β – under repeated mast-cell activation they overstimulate matrix metalloproteinases (MMP-9) while driving the deposition of thin, disorganised collagen that is mechanically weaker than normal tendon tissue.21
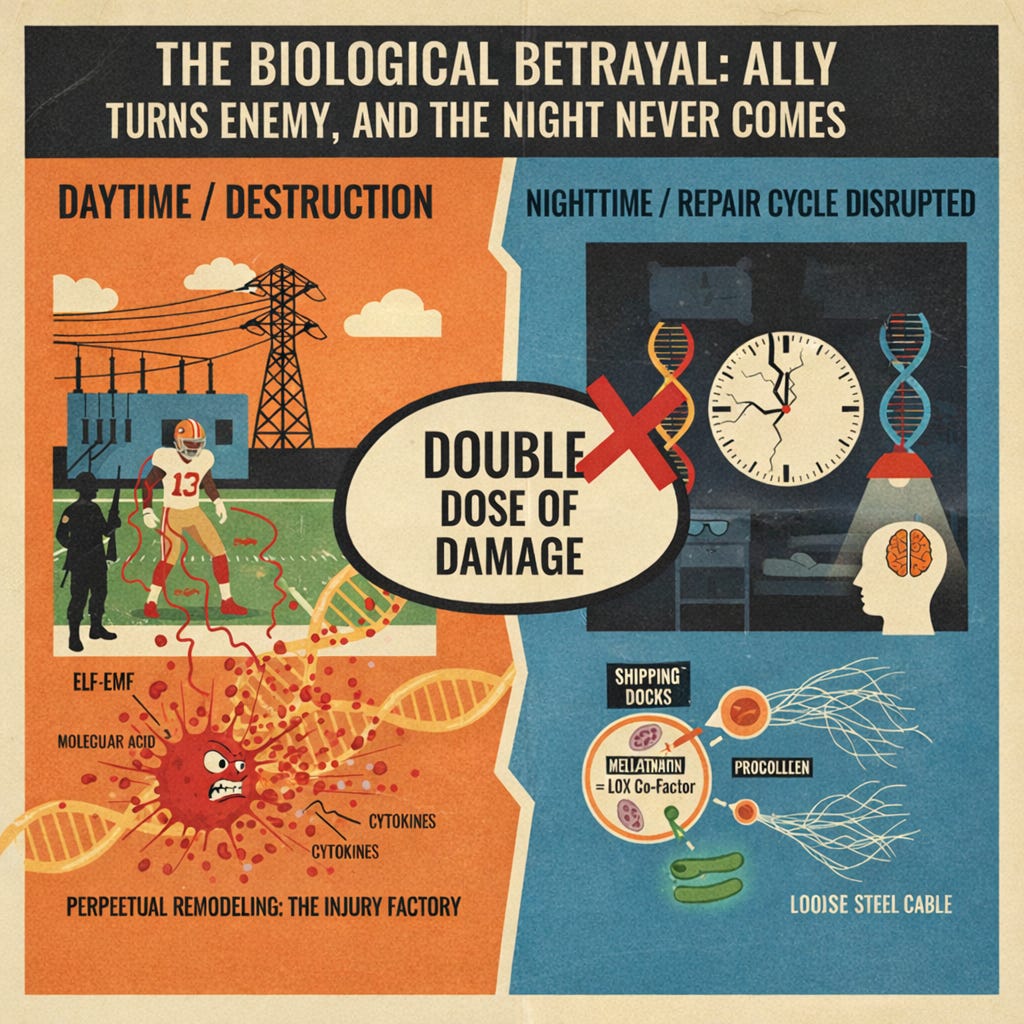
The net effect is simple: every time a player steps into the practice facilities, thousands of microscopic mast-cell activations are setting the stage to remodel the tendons and ligaments into inferior, scar-like material. Do this for years and you no longer have the resilient tissue an NFL athlete needs—you have frayed, overstretched tissue just waiting for the next cut or block to give way. That’s exactly why the same names—George Kittle’s recurring calves, Deebo Samuel’s hamstrings, Trent Williams’ endless soft-tissue battles—keep showing up on the injury report season after season. The scaffolding is being degraded from the inside, day after day.
Pathway 4: Chronic Immune Suppression
Andrew Marino’s 1993 paper “Electromagnetic fields, cancer, and the theory of neuroendocrine-related promotion” laid out a theoretical framework that has aged remarkably well. His neuroendocrine theory (NET) proposed that weak ELF fields, far below thermal limits, act as a persistent environmental stressor detectable by the nervous system. Marino argued that the body transduces these fields into biological signals received by the central nervous system, triggering adaptive hormonal responses that, under chronic exposure, could impair immune surveillance—allowing cells that should be cleared to slip through the cracks.
To test his theory, Marino evaluated the existing epidemiological evidence on EMF-exposed human populations and found that the pattern of increased cancer risk, particularly in children and certain adult populations, was consistent with his neuroendocrine stress hypothesis. While the implications he drew were about long-term cancer risk, the underlying mechanism—chronic HPA axis activation—has more immediate relevance for an NFL athlete.
The body reads these signals the same way it reads any chronic threat, keeping the hypothalamus–pituitary–adrenal (HPA) axis on high alert. Cortisol, our primary stress hormone, climbs to meet the “threat.” While this hormone is essential for alertness, it is inherently catabolic.13
In practical terms, natural killer cells and T-lymphocytes become less aggressive, cytokine balance tilts toward inflammation rather than repair, and cells that would normally be cleared slip through the cracks. Marino's framework predicted exactly this kind of immunosuppressive cascade as the mechanistic link between chronic EMF exposure and disease—a prediction that subsequent research into stress physiology has only strengthened.
For an NFL athlete, this doesn’t mean cancer tomorrow. It means the body is stuck in a low-grade “fight-or-flight” state every single day they train or recover inside the substation’s shadow. Recovery is slower, inflammation lingers longer, and the immune system is too busy reacting to the invisible stressor to fully repair tendons and ligaments. Over a decade, that subtle immune downgrade contributes to the soft-tissue fragility we see on the injury report year after year.
But there is a secondary, hormonal trap hidden within this chronic stress. By keeping the HPA axis "on", the ELF fields prevent the cortisol drop that is supposed to happen at dusk. This lingering cortisol does more than just distract the immune system; it acts as a chemical signal that blunts the body's evening repair shift. As we will see in Pathway 5, a body that is constantly fighting perceived threats is physically incapable of building the resilient tissue it needs at night.
Pathway 5: Circadian Disruption
While the substation’s ELF fields create a unique environmental stressor for the 49ers, the broader mitochondrial and circadian disruptions they exacerbate are a league-wide concern that’s rapidly shedding its fringe label. Blue light from LED lighting, and screens in facilities and players personal devices—emitting high-intensity 450–495 nm wavelengths—mimics daylight, tricking the body’s master clock (the suprachiasmatic nucleus) into delaying melatonin release.
In the NFL, where late-night film sessions and cross-country flights are routine, this hits hard. Players are adapting, though—once considered “woo-woo” blue blocker glasses are now mainstream. Quarterback Brock Purdy swears by them as a critical part of his wind-down routine for his nine hours of sleep, crediting the amber lenses for deeper recovery. Players across the league have been spotted in blue blockers during travel, noting sharper focus and less fatigue. Head coach Kyle Shanahan’s staff incorporates red-light therapy pods, mimicking red and infrared rays from the sun which have a critical role in mitochondrial function (upregulating activity by 20–50% to jumpstart cellular energy production and hydration).
These adaptations, once dismissed as pseudoscience and relegated to fringe biohacker culture, are gaining traction because the data is stacking up—especially in the wake of the 2017 Nobel Prize in Physiology or Medicine awarded to researchers who discovered the molecular mechanisms controlling the circadian rhythm. Building on this, a landmark 2020 study in Nature Cell Biology by Joan Chang et al. revealed that tendon collagen synthesis and cross-linking are not constant; they are strictly managed by the body’s internal clock.
Your body does not build steel cable tendons while you are training; it builds them while you sleep.
During the night, the circadian rhythm initiates a critical transition from catabolic breakdown (the wear from training) to an anabolic phase of structural maturation. The body clears fragmented proteins and reorganizes new collagen into high-capacity fibers.
But this repair cycle requires a functioning cellular logistics chain. The molecular clock genes (Bmal1) physically regulate the export of new collagen out of the cell. At night, the clock triggers the rhythmic expression of proteins that package and move collagen through the endoplasmic reticulum—the cell’s internal assembly line and shipping hub—and facilitate the recycling of fibers left behind by the day’s practice. If this internal clock is disrupted by the substation’s fields and evening blue light, the raw materials for repair remain trapped inside the cell, never reaching the tendon matrix where they are needed.
While the clock manages the logistics, melatonin manages the environment. Beyond its role in sleep, melatonin is the body’s most potent endogenous antioxidant. It acts as a chemical shield, neutralizing the reactive oxygen species (ROS) and clearing the oxidative debris generated by the day’s training and the substation’s ELF fields.
This nightly cleanup is essential for autophagy (the clearing of damaged proteins) and apoptosis (the clearing of cells damaged beyond repair). When the HPA axis is dysregulated and melatonin is suppressed, the oxidative stress from the day continues through the night, and repair is compromised. In this environment, the collagen maturation process breaks down at multiple points. Oxidative stress suppresses the transcription of procollagen genes while simultaneously upregulating matrix metalloproteinases (MMPs)—the enzymes that degrade existing collagen. The result is a double hit: less new collagen is being made, and more of what exists is being chewed up. Without adequate raw material reaching the extracellular matrix, even fully functional Lysyl Oxidase (LOX)—the enzyme that forges the covalent cross-links giving tendons their steel-cable strength—has nothing to work with.
The result is that the players remain trapped in a state of perpetual remodeling, where tissue degradation during practice outpaces structural stabilization at night. Their connective tissue never reaches the density required for elite performance—physically incapable of handling the 2,000+ pounds of peak force generated during an NFL-caliber plant-and-cut.
In the athletic world, emerging research highlights the critical role of circadian rhythms in sports performance, recovery, and injury prevention. A 2023 narrative review in Heliyon emphasizes how circadian rhythm influences athletic output, hormonal regulation (e.g., cortisol and testosterone cycles), immune function, and even injury risk, underscoring the need to align training, recovery, and sleep with natural biological clocks. Similarly, the 2023 International Olympic Committee (IOC) consensus statement on Relative Energy Deficiency in Sport (REDs) addresses how disruptions to energy availability and physiological systems—including circadian factors—can impair performance and health in athletes.
This growing awareness has led some NFL teams to experiment with bio-adaptive or tunable lighting in locker rooms and training facilities to help optimize circadian rhythms. Over the last decade, several teams have installed adjustable LED systems that shift from blue-enriched, bright “daylight” lighting before games or practices (to suppress melatonin and boost alertness) to warmer, dimmer tones afterward (to promote melatonin release and faster sleep onset). The goal is to align players’ internal clocks with demanding schedules—like late games or travel—potentially improving short-term performance, recovery, and reducing fatigue-related risks. While formal studies on these setups in pro athletes are limited, showing mixed results, anecdotal reports suggest benefits like reduced headaches and better mood, and the practice draws from proven circadian-shifting techniques used for night-shift workers.
These interventions represent clever biohacks aimed at short-term performance gains under the rigid constraints of the NFL schedule—late kickoffs, cross-country travel, and film sessions that often extend into the evening. However, by aggressively suppressing melatonin during pre-game windows and forcing artificial phase shifts, they may not fully support long-term health. Chronic reliance on such tactics could exacerbate overall circadian misalignment, perpetuate melatonin suppression, heighten oxidative stress, and contribute to the very soft-tissue fragility we’ve been discussing—where daytime breakdown outpaces nighttime repair. The science suggests that true resilience requires honoring natural rhythms more consistently, rather than constantly overriding them for game-day edges.
The Perfect Storm: A Double Dose Unique to Santa Clara
The EMF concern may be on a similar trajectory—starting as locker-room banter, now bubbling into mainstream discourse. Take YouTube-based NFL commentator Chase Senior’s viral October 2025 tweet, which racked up nearly 4 million views: overlaying a Google Earth map of Levi’s Stadium and the adjacent electrical substation he echoed Feliciano’s quip and tied it to rising EMF awareness around cell phones and wireless tech. Replies ranged from skeptical jabs to earnest shares of studies, proving this “joke” is landing beyond wellness circles. My own X post on Jan 6th, is on its way to 10 million views, and in the wake of the 49ers most recent injuries, this time the commentary is much more amenable to the hypothesis.
In Santa Clara, the ELF fields amplify the process initiated by blue light, adding to the suppression of melatonin, and locking players into a vicious cycle of mitochondrial dysfunction—daytime ETC scrambling, calcium flooding, and mast cell activation, meets nighttime immune modulation, circadian clock dysfunction and cellular repair failure. Players get a double dose of damage, turning general vulnerabilities into a systemic, chronic parade of injuries.
Putting It All Together: Why the 49ers Break While Others Bend
We’ve now explored the full cascade of mechanisms through which chronic exposure to these extremely low-frequency magnetic fields—unique to the 49ers’ Santa Clara facilities—can undermine the structural integrity of tendons and ligaments.
The science paints a compelling, if unsettling, picture: a subtle, invisible environmental stressor unique to Santa Clara that compounds widespread NFL vulnerabilities—high training loads, explosive physical demands, and the relentless grind of a 17-game season—into a systemic fragility that no turf upgrades, scheme adjustments, or elite recovery resources have overcome. And when layered atop league-wide challenges like circadian disruption from artificial light and late-night routines, the result is a perfect storm no other team has to navigate at this intensity.
But if this hypothesis holds—and the biological fingerprint matches the injury pattern with eerie precision—is there any way forward? Is the team doomed to keep breaking, or can knowledge and targeted countermeasures turn the tide?
The substation isn’t going anywhere... but that doesn’t mean the 49ers have to accept fragility as their fate.
(To be continued in Part 4: “The Substation Isn’t Moving—But the 49ers Don’t Have to Keep Breaking.” Subscribe to get notified.)
Appendix A — Key Studies & Reviews
7. Mast-cell degranulation by ELF Primary researcher(s): Olle Johansson
Significance: Demonstrates that low-level electromagnetic fields can trigger the degranulation of mast cells in the skin and connective tissues, releasing inflammatory mediators.
Source: ResearchGate / Karolinska Institute
13. Electromagnetic fields, cancer, and the theory of neuroendocrine-related promotion
Primary researcher(s): Andrew Marino
Significance: Proposes the neuroendocrine theory (NET) as a mechanism linking chronic EMF exposure to cancer risk. Marino theorized that ELF fields act as environmental stressors detected by the nervous system, triggering HPA axis activation and impairing immune surveillance. He evaluated this framework against existing epidemiological studies of EMF-exposed populations and found the patterns consistent with his hypothesis.
Source: Bioelectrochemistry and Bioenergetics, Volume 29, Issue 3, February 1993, Pages 255-276
14. Melatonin suppression by blue light
Primary researcher(s): Reiter & Tan
Significance: Reviews the synergistic effect of artificial light at night on the pineal gland, leading to reduced melatonin and increased oxidative stress.
Source: Journal of Experimental and Integrative Medicine
18. Mast cell histamine loosens tissue matrix
Primary researcher(s): Michael FY Ng
Significance: Explains how the release of histamine from mast cells increases vascular permeability and alters the local tissue environment, contributing to structural weakening.
Source: International Wound Journal
19. The IL-4/IL-13 Axis in Fibrosis and Scarring
Primary Researcher(s): Nguyen JK, Jagdeo J, et al.
Significance: Summarizes how overactive IL-4 and IL-13 signaling triggers a pathological state of “over-repair.” Specifically, these cytokines drive fibroblasts to prioritize the rapid deposition of Type III collagen (procollagen 3α1), which serves as an early marker of active fibrosis and results in disorganized, mechanically inferior tissue compared to healthy Type I collagen.
Source: Archives of Dermatological Research / PMC7008089
20. Mast cell tryptase/elastase degrade collagen/elastin
Primary researcher(s): Pejler G, et al.
Significance: Shows how proteases stored in mast cells act as “molecular scissors,” directly breaking down the protein strands that provide tendons with their tensile strength.
Source: Blood Journal
21. Mast cell VEGF/TGF-β overstimulate MMP-9
Primary researcher(s): Millar NL, et al.
Significance: Research into tendinopathy showing that chronic mast cell activation upregulates Matrix Metalloproteinases (MMPs), which “remodel” the tendon into a mechanically inferior state.
Source: Faculty Reviews
22. Circadian rhythm controls tendon collagen synthesis/cross-linking
Primary researcher(s): Chang, Joan
Significance: A landmark study proving that the “tendon clock” dictates the timing of collagen formation; disruption of this clock prevents the stabilization of new tissue.
Source: Nature Cell Biology
23. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts
Primary Researcher(s): Siwik DA, Pagano PJ, Colucci WS.
Significance: Directly demonstrates that reactive oxygen species (H₂O₂ and superoxide) suppress procollagen gene transcription while simultaneously upregulating matrix metalloproteinases (MMP-2, MMP-9, MMP-13). This “double hit” — less collagen synthesis, more collagen degradation — was reversed by antioxidant treatment, establishing the mechanistic link between oxidative stress and extracellular matrix breakdown.
Source: American Journal of Physiology - Cell Physiology. 2001 Jan;
23a. Reactive Oxygen Species and Tendinopathy: Do They Matter?
Primary Researcher(s): Bestwick CS, Maffulli N.
Significance: Reviews evidence suggesting that elevated ROS impairs collagen synthesis and contributes to tendon pathology.
Source: British Journal of Sports Medicine
24. Lysyl Oxidase: Properties, Specificity, and Biological Roles
Primary Researcher(s): Kagan HM, Li W.
Significance: The definitive review on Lysyl Oxidase (LOX), explaining its role as the “master builder” of the extracellular matrix. It details how LOX catalyzes the oxidative deamination required to forge the covalent cross-links that turn individual collagen strands into high-tensile fibers.
Source: Journal of Cellular Biochemistry
25. LOX-mediated crosslinks fine-tune collagen molecular dynamics
Primary Researcher(s): Dillon S, Clark J, Duer MJ.
Significance: A landmark 2025 study using solid-state NMR to prove that a lack of LOX-mediated cross-linking (KO) produces “friable, dissociated fibrils” that are mechanically compromised. It demonstrates that without proper cross-linking, collagen cannot handle cell-applied forces, leading to a breakdown in mechanosignaling and tissue resilience.
Source: bioRxiv (2025)
26. Narrative review: The role of circadian rhythm on sports performance, hormonal regulation, immune system function, and injury prevention in athletes
Primary researcher(s): Nobari H., Azarian S., Saedmocheshi S., Valdés-Badilla P., García Calvo T.
Significance: This comprehensive review details how the disruption of circadian rhythms (often caused by environmental stressors like artificial light or EMFs) sabotages the hormonal regulation of cortisol and melatonin. It establishes the link between “circadian misalignment” and the suppression of the immune system and nighttime tissue repair, directly explaining why athletes exposed to environmental stress fail to recover from soft-tissue strain.
Source: “Narrative review: The role of circadian rhythm on sports performance, hormonal regulation, immune system function, and injury prevention in athletes.” Heliyon, 9(9), e19636.
27. 2023 IOC Consensus Statement on Relative Energy Deficiency in Sport (REDs)
Primary researcher(s): Mountjoy, M., et al.
Significance: This is the global standard for understanding how “problematic Low Energy Availability” (LEA) leads to the syndrome known as REDs. It provides the physiological evidence that when a cell’s energy metabolic rate drops, musculoskeletal health and immunity collapse, leading to a massive increase in injury risk. This supports your theory that the Metcalf substation creates an “Environmental REDs” state in 49ers players.
Source: Mountjoy, M., et al. (2023). “2023 International Olympic Committee’s (IOC) consensus statement on Relative Energy Deficiency in Sport (REDs).” British Journal of Sports Medicine, 57(17), 1073-1097.
28. NFL Teams are Trying to Win More Games with Lights